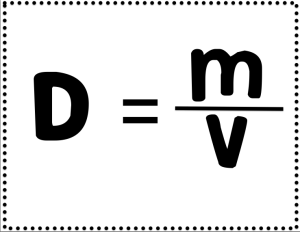Tuesday, March 21, 2017
Science Magic Show!
A couple of weeks ago over 10 learners invited their families in to observe some Science Magic. Learners had performed these trick in front of a smaller audience in class, but wanted to share the fun with a larger group of people!
Over 30 people watched as learners performed tricks that could only be explained through science!
Take a look at what the students were able to do!
Plants beginning to grow!
We are excited to see that many of our plants and "Chia" houses are beginning to grow! It was fun to watch as our learners took care of their plants.
We will begin to HARVEST these plants soon so that we can look at them a little closer under a microscope.
We even grew some mold that we can look at!
We will begin to HARVEST these plants soon so that we can look at them a little closer under a microscope.
We even grew some mold that we can look at!
Thursday, March 2, 2017
Beginning to explore cells!
As we begin to explore how atoms and molecules join to form larger things, some of them living, we will do extensive exploring of different types of cells and their function.
To kick off the unit, learners are planting a variety of seeds in order to have live specimens that we can examine at a later date.
To kick off the unit, learners are planting a variety of seeds in order to have live specimens that we can examine at a later date.
One of the fun ways in which we are planting seeds is by making seed houses out of sponges! You can see some of the learners work here:
Wednesday, February 22, 2017
Magical Science Videos
You can watch a lot of the action from Friday at our classroom YouTube Channel at:
https://www.youtube.com/channel/UCUoRg354TSEmkqZj4O96zWg
https://www.youtube.com/channel/UCUoRg354TSEmkqZj4O96zWg
Thursday, February 16, 2017
Preparing for our magic show!
Learners are gathering materials and practicing their science magic tricks! I am excited to see what they do tomorrow!
Thursday, February 9, 2017
Heavy Balloon
Wednesday, February 8, 2017
Crushing bottle magic!
This demonstration briefly shows how molecules are effected by changes in temperature within a closed system.
Oobleck!
Bartholomew and the Oobleck!
Back in 1949, Dr. Suess wrote a book about crazy stuff falling from the sky in the Kingdom of Didd. This stuff was created by the king's wizards and was stick and gooey and caused all sorts of problems. They called the stuff Oobleck.
Teachers for years have been creating their own versions of Oobleck for use in science classrooms as learners explore the different states/phases of matter.
Our version is simply made by mixing corn starch and water.
The great thing about the mixture is that learners can feel how the substance acts like a solid when they squeeze the molecules together, yet the substance flows freely like a liquid when pressure is released! It really is a lot of fun to play with!
Thursday, February 2, 2017
Density Labs - Details
Learners spent over a week working in our STEM labs at the 2-8 building working on finding the mass, volume, and density of various materials.
Below is a summary of a few of the labs:
Reading with a twist
The other day, we spent a little time reading from the book Atoms and Molecules by Spilsbury.
To add a little fun to the activity, we played a little game inspired by a radio show that used to run on 700 WLW. They called it "Sports or Consequences" and folks would call up and try to "Stump the Chump" with trivia questions pertaining to Cincinnati sports history.
If the hosts got the questions correct, they would yell..."We don't, We don't, We don't mess around. Hey!"
Our learners had fun trying to stump their friends with interesting facts from the book!
To add a little fun to the activity, we played a little game inspired by a radio show that used to run on 700 WLW. They called it "Sports or Consequences" and folks would call up and try to "Stump the Chump" with trivia questions pertaining to Cincinnati sports history.
If the hosts got the questions correct, they would yell..."We don't, We don't, We don't mess around. Hey!"
Our learners had fun trying to stump their friends with interesting facts from the book!
Disappearing Ball - A demonstration in density
Learners spent a lot of time over the last few weeks exploring and calculating the density of many different things.
This was a small demonstration of density that we did in class...
This was a small demonstration of density that we did in class...
Thursday, January 26, 2017
Mystery Materials - Chemical Reaction
As learners continued to learn about chemical reactions, they took part in a lab in which they were mixing "unkown" materials.
As they are beginning to understand, sometimes when you mix different types of materials, a chemical reaction will take place and a new substance will be formed. In this case, the resulting material resembled and acted like a bouncy ball.
Physical vs. Chemical Change - CO2 Extinguisher
As we look at chemical vs. physical changes, we are beginning to explore how to tell the difference.
5 Changes that typically show a chemical reaction:
5 Changes that typically show a chemical reaction:
- A gas is formed
- A new substance is formed
- Heat and/or light is generated or lost
- A new smell is formed
- A dramatic color change occurs.
Below is a demonstration done in class, mixing baking soda and vinegar. The resulting reaction produced Carbon Dioxide which I used to put out candles.
Tuesday, January 10, 2017
Mystery Mass Rods Lab
As part of our exploration on density, learners used an equal mass rods kit to discover that rods of various volumes can still have the same mass if their densities are different.
Watch two of our learners discover this...
Monday, January 9, 2017
Density Labs
Matter and Motion
Atoms, Molecules and Density
This week, we will be taking a closer look at atoms, molecules and how they are positioned in terms of density. Learners will have an opportunity to learn how to calculate density through a series of 8 labs. Each lab focuses on at least one part of the density calculation.
Each lab will be highlighted in further blog posts as the week continues.
Ohio State Science Standards covered are highlighted below:
Wednesday, January 4, 2017
Matter and Motion Videos
A large part of our Matter and Motion unit will involve studying atoms and molecules. As these are VERY hard to see...studying them through video and animation are the best and sometimes only route to understanding.
Take a look at our playlist of videos on YouTube. These will be used in class multiple times.
Take a look at our playlist of videos on YouTube. These will be used in class multiple times.
Subscribe to:
Comments (Atom)